Author Guidelines
INTRODUCTION
AMJAM is an open-access journal publishes Original article, Review article, Brief Research, Case report, Special article (Invited by editors), Editorial, and Letter to the Editor in health sciences and alternative medicine. AMJAM publishes 3 times a year in April (Issue 1), August (Issue 2) and December (Issue 3). All submitted articles will be evaluated using double-blinded review process by 3 reviewers.
DOWNLOAD AUTHOR GUIDELINES PDF HERETYPES OF ARTICLES
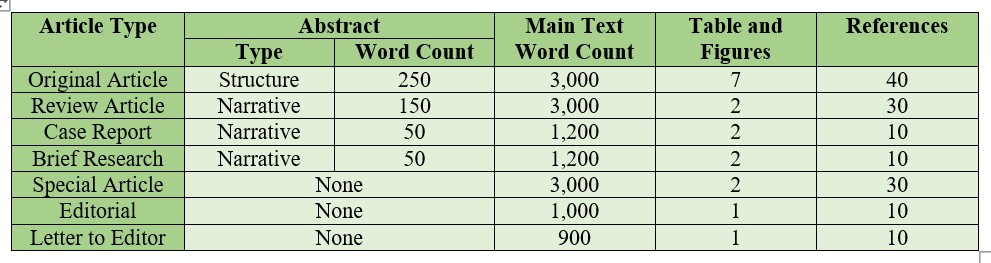
Original Article should include a title page, a structured abstract of no more than 250 words, a text of no more than 3,000 words, no more than 7 tables and figures, and no more than 40 references. Original Article Template
Review Article should include a title page, a narrative abstract of no more than 150 words, a text of no more than 3,000 words, no more than 2 tables or figures, and no more than 30 references. Review Article Template
Case Report should include a title page, a narrative abstract of no more than 50 words, a text of no more than 1,200 words, no more than 2 tables or figures, and no more than 10 references. Case Reports Template
Brief Research should include a title page, a narrative abstract of no more than 50 words, a text of no more than 1,200 words, no more than 2 tables or figures, and no more than 10 references. Brief Research Template
Special Article (Invited by editors) should include a title page, a text of no more than 3,000 words, no more than 2 tables or figures, and no more than 30 references. Abstract nor keywords are not required. Special Article Template
Editorial should include a title page, a text of no more than 1,000 words, no more than 1 table or figure, and no more than 10 references. Abstract nor keywords are not required. Editorial Template
Letter to the Editor should not exceed 900 words, no more than 1 table or figure, and no more than 10 references. Abstract nor keywords are not required. Letters to the Editor Template
SUMMARY TABLE OF THE REQUIREMENTS FOR DIFFERENT ARTICLE TYPES
REQUIRED FILES/DOCUMENTS FOR SUBMISSION
- Author submission agreement form
- Cover letter
- Title page, manuscript, figures and tables
- Copy of ethics committee/institutional review board's certificate of approval
- Checklist of Standards of Reporting based on the study type (see below)
- Graphical abstract (if available)
MANUSCRIPT PREPARATION
Standards of Reporting
Authors are required to use the relevant research reporting guidelines for the study type provided by the EQUATOR (Enhancing the Quality and Transparency of Health Research) Network when preparing their manuscript. Authors must adhere to these guidelines and submit a completed checklist of Standards of Reporting based on the study type. The commonly used reporting guidelines with downloadable checklists are:
- Randomized controlled trials (RCTs): CONSORT (Consolidated Standards of Reporting Trials) - for clinical trials
- Systematic reviews and meta-analyses: PRISMA (Preferred Reporting Items of Systematic Reviews and Meta-analyses) - for systematic reviews and meta-analyses
- Observational studies in epidemiology: STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) - for cross-sectional, case-control, and cohort studies
- Qualitative research: SRQR (Standards for Reporting Qualitative Research) - for all studies involving qualitative research
- Economic evaluations: CHEERS (Consolidated Health Economic Evaluation Reporting Standards) – for health economic research
- Animal pre-clinical studies: ARRIVE (Animal Research: Reporting of In-Vivo Experiments) - for all studies involving laboratory animals
- Guidelines for case reports: CARE (The Case Report) - for case reports
Authors who are not fluent in English should have their manuscript checked by a native speaker of English and/or an editing service that provides such assistance. Please contact the editorial office if author would like to receive suggestion on the editing service. Manuscripts that do not follow the required format or are poorly prepared will be rejected or returned to the author for revision and resubmission. In the submitted cover letter, authors must state that "All authors have significantly contributed to the research". For the article types that require reviewers, authors must suggest five reviewers who are not in the same institutions as authors' and have no conflict of interest in the manuscript preparation and publication.
All submitted manuscript must include an author submission agreement and the institutional ethical approval certificate. Single space the entire manuscript, including title page, abstract, body text, acknowledgements, references, tables, and figure legends. Use left justification only, so that the right margin is ragged. Number pages consecutively, beginning with the title page. Use Times New Roman font and set the font size to 12 points (for tables as well as text) unless otherwise indicated in each article template. All numbers published in AMJAM will be in Arabic numbers. Each component of the article should begin on a separate page, as follows: title page, abstract, body text (including figures, figure legends and tables), references and appendices. All these components must be in a single file. The contents of each section should conform to the guidelines below.
Studies in humans and animals
If the work involves the use of human subjects, the author should ensure that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Authors should include a statement in the manuscript that informed consent was obtained for experimentation with human subjects. The privacy rights of human subjects must always be observed. All animal experiments should comply with the ARRIVE guidelinesand the authors should clearly indicate in the manuscript that such guidelines have been followed.
Title page
The title page should include the following information: (1) the title of the manuscript; (2) the names of the author(s), including each author's highest academic degree or professional certification; (3) the departmental and institutional affiliation of each author, including city, state or province, and country; (4) the name, address, telephone number, fax number, and e-mail address of the author responsible for correspondence; (5) if relevant, a statement about any previous presentation of the data or findings in a preliminary report or abstract; (6) an abbreviated title of not more than 45 characters (including spaces), to be used as a running head and for online searching; and (7) a word count for the body of the text (excluding the abstract, data availability, financial and conflict of interest statements, compliance with ethics requirements, acknowledgements, author contributions and the references). The names of the authors must be spelled and written identically as they appear in the ORCID database.
Authors must list all relevant affiliations to attribute where the research was approved and/or supported and/or conducted. For non-research articles, authors must list their current institutional affiliation. In cases where an author has moved to a different institution before the article has been published, they should list the affiliation where the work was conducted, and the current affiliation and contact details should be listed in the acknowledgment section. Change of affiliation alone is not a valid reason to remove an author from a publication if he or she meets the authorship criteria.
Abstract
Original Article should include a structured abstract of no more than 250 words. The following headings are suggested: Introduction/Objective(s), Design, Methods (or Interventions), Results, Conclusions, and Keywords (3-5 words or phrases). If this list of headings is inappropriate, variations are permitted: for example, a study that involved no intervention would use the heading "Methods" rather than "Intervention". For brevity, parts of the abstract can be written in phrases rather than complete sentences (e.g., "Design: Retrospective cohort study" or "Design: Before-after trial").
Body text
The main sections and subdivisions of the body text should be indicated by side heads flushed with the left margin and two lines above the text. Keep Methods, Results, and Discussion distinct and separate. The Methods section should provide details sufficient to allow others to reproduce your experiment. Methods should not be described or restated in figure legends or table notes, but must be all together in the Methods section. The Results section contains the previously unpublished data derived by this application of your methods, without commentary. The Discussion section contains your interpretation of the reported data and comments on its meaning. There should be no separate section labeled "Conclusion." Duplicating in the text data that have been provided in tables or figures should be avoided (minimal duplication, for emphasis or clarity, is acceptable). Duplication within the text should be avoided; for example, the Discussion section should not restate all the findings that have been presented in the Results section and/or in tables and figures.
Nomenclature and units
All measurements and data should be given in SI units where possible, or in other internationally accepted units in parentheses throughout the text. Illustrations and Tables should use conventional units, with conversion factors given in legends or footnotes.
Acknowledgments and other statements
Descriptions of the following statements should be provided after body text and before the references.Research Data Policy
AMJAM encourages the sharing of research data to promote transparency, reproducibility, and the advancement of knowledge within the academic community. We recognize the importance of making data accessible to other researchers while respecting ethical and legal considerations. To this end, we have established the following guidelines for the inclusion of research data statements in submitted manuscripts:Data Availability Statements: Authors are required to include a Data Availability Statement in their manuscripts. This statement should clearly outline where the data supporting the findings of the study can be accessed or explain why the data cannot be shared. Below are examples of acceptable Data Availability Statements:
- Data Available in a Public Repository: The datasets generated during and/or analyzed during the current study are available in the [NAME] repository, [PERSISTENT LINK TO DATASETS].
- Data Available on Request: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
- No Data Available: No datasets were generated or analyzed during the current study.
Financial support: Information on financial support should be provided. The authors should list all sources of financial support for the work, including any financial arrangement with a company whose product is related to the study. If there was no financial support, that too should be stated.
Example: Financial support: The XXX Project is supported by the Thai Ministry of Health. Additional support for this study was provided by Becton-Dickinson.
Financial support: None reported.
Conflict of interest: This should be stated. If the manuscript is accepted for publication, the disclosures will be published. The authors must list the name of each contributing author and any potential conflicts of interest for each author for the previous three years; if no potential conflict exists, that too should be stated.
Example:
Potential conflicts of interest: K.L.H. reports having consulted for and having received grant support from Astellas and reports having received an honorarium from Cubist before starting employment with the New York Department of Public Health in 2009. Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
Compliance with Ethics Requirements: This must be stated in the manuscript. Authors should include information regarding compliance to ethical standards and informed consent if the research involved human participants, and a statement on welfare of animals if the research involved animals. The name of ethics committee, institutional review board which approves the study must be stated along with the approval number.
Acknowledgements: Any individuals who have contributed to the article (e.g. general supervision, acquisition of funding, study design, data collection, data analysis, technical assistance, formatting-related writing assistance, scholarly discussions which significantly contributed to developing the article, etc.), but who do not meet the criteria for authorship should be listed by name and affiliation in the Acknowledgments section. It is the responsibility of the authors to notify and obtain permission from those they wish to identify in this section. The process of obtaining permission should include sharing the article so that those being identified can verify the context in which their contribution is being acknowledged.
Groups of persons who have contributed materially to the article but whose contributions do not justify authorship may be listed under such headings as "clinical investigators" or "participating investigators," and their function or contribution should be described - for example, "served as scientific advisors," "critically reviewed the study proposal," "collected data," or "provided and cared for study patients." Because readers may infer their endorsement of the data and conclusions, these persons must give written permission to be acknowledged.
Any assistance from AI tools for content generation (e.g. large language models) and other similar types of technical tools which generate article content, must be clearly acknowledged within the article. It is the responsibility of authors to ensure the validity, originality, and integrity of their article content. Authors are expected to use these types of tools responsibly and in accordance with our editorial policies on authorship and principles of publishing ethics.
Author contribution statement: This should be provided in the manuscript. Each author is expected to have made substantial contributions to the conception or methodology of the work; or the acquisition, analysis, or interpretation of data; or have drafted the work or substantively revised it. When writing the author contribution statement, the authors should refer to the CRediT author statement guidelines and use the CRediT taxonomy.
Example:
Anthony Brae and Britney Cross.: Conceptualization, Methodology, Software. Ann Health.: Data curation, Writing- Original draft preparation Catherine Dough and Donn Elf.: Visualization, Investigation, Writing- Reviewing and Editing.
References
Use the Style Guide of the American Medical Association (AMA) as a reference. References should be cited consecutively in the text, with superscripted numbers placed outside periods and commas and inside colons and semicolons. References cited only in tables or figure legends should be numbered as though all were cited at the point at which the table or figure was first mentioned.A paper that is "in press" may be included in the reference list if it has been accepted for publication. Citations such as "in preparation," "submitted for publication," "unpublished data," and "personal communication" should be given in parentheses in the text only, including the names of all individuals to whom the information should be attributed, as well as each person's highest academic degree and the month and year of the information's origin. For personal communications, specify whether the communication was written or oral.
At the end of each manuscript, list the references in numerical order, single spaced, according to the order they are cited in the text. If there are 7 or more authors, list the first 3 authors' names, followed by "et al"; otherwise, list all authors. Abbreviations of journal names should conform to Index Medicus or MEDLINE. Unlisted journals should not be abbreviated. Authors are responsible for bibliographic accuracy. Journal titles should be cited as they existed at the time of publication. Format references according to the style given in the AMA Manual of Style, 10th Edition.
AMA CITATION STYLE GUIDE.
Journal articles (examples)
Pittet D, Simon A, Hugonnet S, Pessoa-Silva CL, Sauvan V, Perneger TV. Hand hygiene among physicians: performance, beliefs, and perceptions. Ann Intern Med. 2004;141:1-8.
Camins BC, Richmond AM, Dyer KL, et al. A crossover intervention trial evaluating the efficacy of a chlorhexidine-impregnated sponge in reducing catheter-related bloodstream infections among patients undergoing hemodialysis. Infect Control Hosp Epidemiol. 2010;31:1118-1123.
Journal article in press (example)
Figueroa P, Johanssen KL, Price FG, et al. Outbreak of Acinetobacter infection in a neonatal intensive care unit. Pediatr Infect Dis J (in press).
Paper presented at a professional meeting (example)
Chen LF, Freeman JT, Sexton DJ, Choi YI, Anderson DJ. NHSN definition of laboratory-detected BSI is overly sensitive for Enterococcus. In: Program and abstracts of the 19th Annual Scientific Meeting of the Society for HealthcareEpidemiology of America (SHEA); March 18–22, 2009; San Diego, CA. Abstract 359.
Book (example)
Heoprich PD. Infectious Diseases. 2nd ed. New York, NY: Harper and Row; 1977.
Chapter in a book (example)
Schaffner W. Psittacosis: ornithosis, parrot fever. In: Beeson PB, McDermott W, Wyngaarden JB, eds. Cecil Textbook of Medicine. 15th ed. Philadelphia, PA: W. B. Saunders; 1979:336-338.
Web page (example)
Clinical laboratory fee schedule. Centers for Medicare and Medicaid Services. https://www.cms.gov/ClinicalLabFeeSched/02_clinlab.asp#TopOfPage. Published 2010. Accessed April 2, 2010.
Tables and Figures
Tables and figures in articles are clear and well configured within article content (are not just copied-pasted from the Excel program).
Tables Prepare tables with the MS Word table editor; text formatted to look like a table by use of tabs and hard returns is not acceptable and will be rejected. Include tables in the same file of the manuscript, not in separate files. Tables should be single spaced. Number tables in the order in which they are cited in the text, and provide a descriptive title for each table.
Every column in a table requires a head that describes the contents of the cells below. The units of measure for all data must be clearly stated in the heads, in the stub (leftmost) column, or in data cells, as appropriate. Do not use vertical lines, and do not use ditto marks for repeated information.
List and define any abbreviations in a note below the table, even if the abbreviations have been defined in the text. Use superscripted letters for footnote designators.
Examples of Table:
Figure The size of image is with a minimum /of 35 x 14 cm. (w x h) using a minimum resolution of 300 dpi. Larger image, please use the same ratio. The file types are PDF or MS Office files.
Examples of Figure:
Graphical abstract Authors can consider providing an original image that clearly represents the work described in the manuscript. Graphical abstract should be submitted as a separate file in the submission system with the requirements below: Size: Image with a minimum of 35 x 14 cm. (w x h) using a minimum resolution of 300 dpi. Larger image, please use the same ratio. Font: Times New Roman File types: PDF or MS Office files Graphical Abstract Template
REVISED MANUSCRIPTS The authors must submit the revised version of their submissions within 30 calendar days of receiving the editorial decision. The extension of this revision period needs to be requested with indicating the expected resubmission date and explaining reasons. This request will be granted based on the editors' judgment. Revision does not mean that the manuscript will be accepted for publication, as the amended submissions could be sent out for reevaluation. In response to reviewers' comments, the authors must ensure that each comment is followed by their revision and/or response. In instances where an author disagrees with a comment or suggestion of a reviewer, please justify the reason. Any associated changes in the manuscript must be highlighted in the revised form of the manuscript to facilitate the process of re-evaluation.
AFTER ACCEPTANCE Upon acceptance, the article will be exported to production to undergo typesetting. once the typesetting is complete, the authors will receive the proofs.
PROOFS One set of page proofs will be sent by email to the corresponding author. Please use this proof only for checking the typesetting, editing, completeness, and correctness of the text, tables, and figures. We will do our best to get your article published quickly and accurately. Therefore, it is important to ensure that all of your corrections are sent back in one communication within 48 hours. Proofreading is solely your responsibility. Note that the publisher may proceed with the publication of an article if no response is received.
WITHDRAWING A MANUSCRIPT To withdraw your manuscript after being reviewed and accepted by the reviewers and editors (but has not yet published), the authors must write a clear and concise letter with explaining reasons as to why the manuscript needs to be withdrawn.
SUBMISSION
Article Type Templates Original Article Template Review Article Template Case Reports Template Brief Research Template Special Article Template Editorial Template Letters to the Editor Template
Checklist: To ensure a smooth review process and maintain consistency in our publication standards, we kindly request that the authors carefully review and adhere to the following Submission Preparation Checklist:
- All submitted manuscripts must include an Author submission agreement form
- The manuscript is an original work and has not been published or is not currently under review with other journals or Conference Proceedings
- The manuscript is in full adherence with all of the publication ethics
- For manuscripts describing a study involving humans or animals, the treatment of subjects was in accordance with ethical guidelines and appropriate institutional review board/ethics committee approval has been obtained
- Permission to reproduce or adapt any copyrighted material from other sources has been obtained and documentation for this permission can be provided
- The English of the manuscript is acceptable and it should be free of grammatical and spelling errors
- The manuscript should be formatted and edited according to the journal template for each type of articles ( Author guidelines)
- The references are formatted correctly and numbered as they appear in the text ( Author guidelines)
Submissions that don't adhere to these guidelines will be rejected or returned to the author prior to the peer review process.



